Warning: Membrane modules are supplied non-sterile. It is the responsibility of the end user to validate the sterilization procedure used for the module as a component in their particular system. All bioreactor systems and tubing sets are different and have different components. Suggestions provided are only to be considered as possible options for configuring the user's particular system. The end user shall ultimately determine the suitability and optimal configuration for their own system.
PermSelect membrane modules can be sterilized one time by steam or dry heat autoclave (up to 121 C), gamma irradiation, and ETO gas. The method selected typically depends on how other components in the system can be sterilized. However, if the user has the option to choose either method presented, the following information should be considered in such choice.
Steam and dry heat autoclave The high temperatures applied with steam and dry heat present a challenge because of the thermal expansion of the different materials that compose the membrane module, and thus several precautions must be taken before high temperature exposure:
- Ensure that the temperature does not exceed 121 C
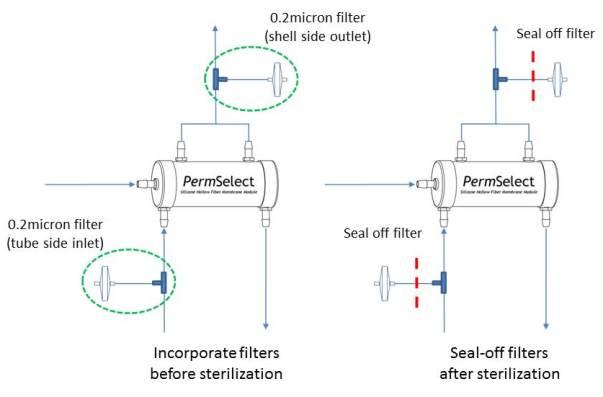
- Ensure that both (shell and tube) sides of the membrane are vented to the autoclave so that pressures cannot build-up and possibly exceed the maximum membrane pressure limitation. This can be accomplished, while maintaining sterility by incorporating a 0.2 micron filter on each side of the membrane module as shown below. The venting filters could be placed on the inlet or outlet of the shell and tube sides of the membrane. The filters shown in the diagram below are placed on the inlet of the shell and tube sides of the membrane. The filters, or tubing leading to the filters should be permanently sealed off after sterilization and prior to using the membrane module.
- Note that if 0.2 micron filters are already part of the intended tubing set configuration, they may be used to vent the membrane provided they allow autoclave gas to freely enter the shell and tube side of the membrane module.
It should be noted that another advantage to incorporating the filters in the tubing set is that it enables testing for integrity of the membrane module in a sterile manner after sterilization. The test method consists of applying a pressurized gas (nitrogen or air) on one side of the membrane and measuring gas permeation across the membrane. Since the permeability of the gas is known, the gas flow permeating the membrane should be within a specified limit if the membrane is intact after sterilization. Please contact us for more details about conducting this test. - Ensure that the tubing connectors on the membrane module are not subject to high stresses during autoclave to prevent bending, loosening, or breaking as the housing components soften at high temperature.
Gamma radiation A number of customers have reported good results with gamma radiation in the 25-40 kGray range. Although minor change in the module housing color (yellowing) is observed, no change in performance has been reported. The advantage of gamma radiation sterilization over heat methods is that the membrane module is not subjected to the high thermal stresses, thus the probability of changes to the integrity on the module is reduced during sterilization.