Oxygen, carbon dioxide, and other gases dissolved in liquids used in the bio-pharmaceutical industry can adversely affect many processes, thus delivering water, drugs, therapeutics, and other liquids that have very low levels of dissolved gases and microbubbles leads to improved process quality and yields.
PermSelect® silicone membrane degassers are very effective for continuous, inline degassing of liquids used in the bio-pharmaceutical industry. Unlike direct vacuum degassing and ultrasonic degassing of liquids, which are batch degassing approaches, membrane degassing using PermSelect® membrane degassers can be accomplished in a continuous mode, inline with other processes such as filtering, dispensing and filling. PermSelect® silicone membranes degassers can be used for degassing and removing micro bubbles in many liquids including water, drug product, therapeutics, gels, and other high viscosity liquids, even at high temperatures. Moreover our membrane degassers can be implemented as disposable (single use) or reusable in durable settings.
For example, when filling a pharmaceutical liquid drug product into vials, bags, syringes, or ampoules, gas bubbles may be created or exposed, which can be falsely detected as an impurity or a foreign particulate matter by automated optical inspection systems, resulting in high false-rejection rate (rejection of acceptable vials or containers). Degassing the pharmaceutical and biologic liquid drug product solution prior to the filling step can eliminate the bubbles which provoke the false rejection, thereby reducing losses due to discarding drug product or time necessary for further inspection.
Similarly, dissolved gases and bubbles in biologics and therapeutics prior to freezing can significantly impact the protein drug product quality attributes, including formation of visible particles, subvisible particles, soluble aggregates, and changes in target protein concentration due to adsorption of the molecule to various interfaces. The dissolved gas in the liquid is rejected and accumulated ahead of the ice-water interface during solidification and the resulting bubbles are incorporated into the growing ice crystal. Formation of ice crystals can contribute to cryoconcentration which also affects reaction rates. Reduction in temperature lowers the rate of degradation reactions (the Arrhenius effect), but cryoconcentration can counteract that through an increase in the concentration of reactants. So reactions such as oxidation can be enhanced, especially when the solubility of oxygen increases as temperature drops while ice formation also excludes gases. Dissolved oxygen at high concentration can be trapped as bubbles along with proteins in the final glassy matrix.
Thus, degassing biologics and therapeutics prior to filling bags, vials, syringes, or ampoules, can eliminate the subsequent problems encountered with freezing.
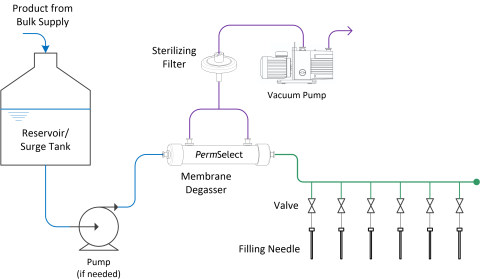
The diagram below illustrates how simple and straightforward it is to include a degassing step at filling of either pharmaceutical drug products to be inspected by optical systems, or therapeutics to be frozen.
Drug Product and Therapeutic Degassing at Filling Lines
Our membrane modules can be sterilized by steam autoclave (including SIP), gamma radiation, EtO and other harsh chemicals. PermSelect membrane degassers are available in FDA compliant materials, including USP Class VI, ISO 10993-5, and FDA CFR 21 compliant materials with low extractables. Prior to shipping, each membrane degasser is individually tested following a rigorous protocol. Our membrane degassers provided to pharmaceutical customers are shipped with a material certificate of compliance (MCC). Contact us for details. Click here to view a white paper on degassing liquid drug product and biologics at filling stations.
The bio-pharmaceutical industry also requires high water quality without contamination. High purity water is required in pharmaceutical processing where conductivity requirements of < 1.3µS/cm are common. To achieve this level of water purity, dissolved carbon dioxide has to be removed in advance of EDI. Moreover, by removing dissolved O2 membrane provide even more benefits; the presence of oxygen can negatively affect the shelf life and stability of some products; PermSelect membrane degassers offer a simple, effective solution to remove dissolved O2 and CO2 in processes where products would be impacted by its presence. PermSelect Membrane Modules may be used to degas water before it is used to make WFI by distillation or RO.
PermSelect Membrane Modules can be used as a compact, chemical-free alternative that operates in closed systems which may help reduce the risk of contaminants from entering the purified water stream.
How to Degas Liquids using PermSelect® Membrane Contactors and Degassers?
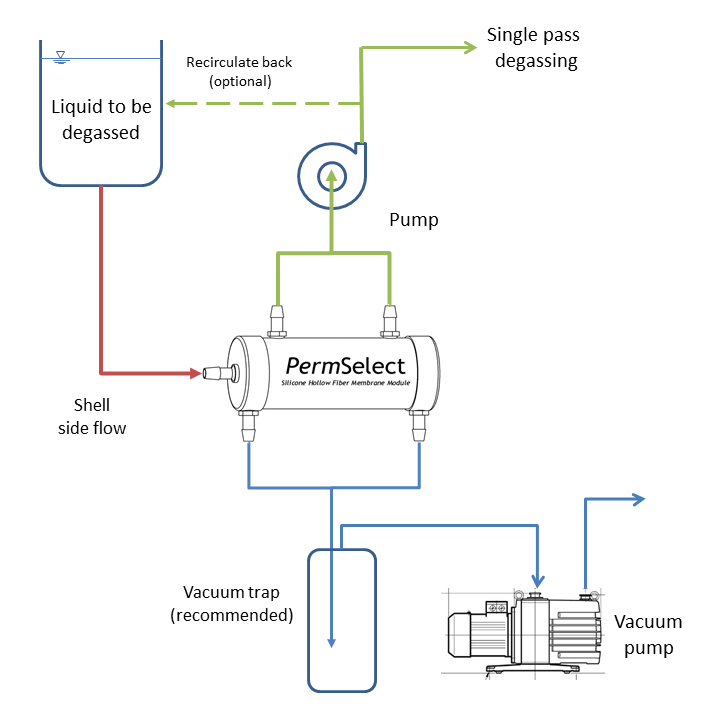
Degassing liquids using PermSelect® degassers and membrane contactors is straightforward as illustrated in the figure below. Liquid with dissolved gases or microbubbles is contained in a reservoir, or a continuous feed is supplied upstream from the membrane contactors or degasser. A pump may be placed in the circuit (upstream or downstream from the membrane contactor) if needed to provide a required flow rate through the system. The simplest method to degas a liquid is to use straight vacuum to remove all dissolved gases from the liquid. As shown in the figure below, liquid enters the center port of the PermSelect® membrane contactor, then flows on the outside of the hollow fibers (shell side) and exits the at side ports (shown on top of the module). Vacuum is applied at the ports on the end caps which provide vacuum to the inside (lumen side) of the hollow fibers. As liquid flows through the hollow fiber bundle, dissolved gases permeate the hollow fiber walls driven by the vacuum in the lumens. Extracted gases flow toward the vacuum pump and degassed liquid exits the side ports of the degasser.
Liquid degassing can be accomplished continuously with a single pass through the degasser or membrane contactor or with multiple passes by recirculating the fluid through a reservoir. The choice will depend on the system design, the capacity of the module to remove gases, and the required level of degassing. Other considerations include liquid-membrane compatibility, and the system fluid pressures. Please view the silicone chemical compatibility chart as an initial substance compatibility guide. Depending on your system pressure requirements, a swapping of liquid flow side and vacuum (a lumen side liquid flow) may be required. The maximum recommended trans-membrane pressure (TMP) for shell side liquid flow is 15 psi. So if your system exceeds this TMP then a lumen side liquid flow (as shown in the figure at the top of this page) is recommended up to 45 psi TMP. Contact an applications engineer, or call +1 (734) 769-1066 to discuss your particular liquid degassing needs.
MedArray provides its PermSelect® membrane contactors and degassers for vacuum liquid degassing directly to researchers, and to industry through original equipment manufacturers (OEM’s) who are interested in integrating degassing solutions in their equipment. We can also customize membrane modules to your specific application. Contact us to discuss your custom application.